FLUORSPAR FROM PAKISTAN
CAF2: CALCIUM FLUORITE by Comprehensive Enterprises, Pakistan.
We can supply fluorspar in lumps according to following: (METALLURGICAL GRADE FLUORSPAR-)
Packing : 1MT P.P JUMBO BAG Mine Location: LoraLai, Balochistan, Pakistan.
|
FLOURSPAR METALLURGICAL GRADE IN PAKISTAN |
||
| Purity of CaF2 | Size/as per request | SUPPLY/MONTH |
| 85%-90% | 10mm-50mm | 300-600MT |
| 80%-85% | 10mm-50mm | 300-1000MT |
| 75%-80% | 10mm-50mm | 300-1500MT |
| 70%-75% | 10mm-50mm | 300-2000MT |
| 65%-70% | 10mm-50mm | 300-2500MT |
|
60%-65% |
10mm-50mm |
300-3000MT |
MINES OF FLUORSPAR
Location : Loralai, Balochistan, Pakistan Loading port: Karachi port,
Estimated deposit per each mines: Above 50000 Metric
TON Possible period of production: Every Month
Production capability :100mt per day /3000mt per months CaF2:80% above, 400MT/month, CaF2:72~80%, 3000MT/month Expected
Delivery : 40days including transit time in sea Production Process (from mining to entire package) :10-15 days – Quality control process, SGS test report before shipment, PCR test report before shipment, Geoscience Advance Research, Laboratories, Government of Pakistan, Islamabad.
FLUORSPAR FROM PAKISTAN
IN PAKISTAN LABORATORY HOWEVER THE PERCENTAGE OF CACO3 AND SIO2 IS SLIGHTLY VARIABLE ACCORDING TO DIFFERENT MINING PLACE. SO PLEASE FIND IT ACCEPTABLE.
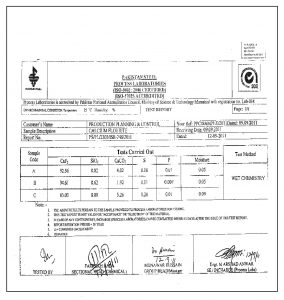
FLUORSPAR FROM PAKISTAN
TEST REPORT FOR FLOURSPAR BY SGS IN PAKISTAN

SGS INSPECTION FOR FLOURSPAR AT OUR WAREHOUSE IN KARACHI



FLOURSPAR PACKED IN JUMBO BAG AT OUR WAREHOUSE IN KARACHI
What is Fluorite?
Fluorite is an important industrial mineral composed of calcium and fluorine (CaF2). It is used in a wide variety of chemical, metallurgical, and ceramic processes. Specimens with exceptional diaphaneity and color are cut into gems or used to make
ornamental objects.
Fluorite is deposited in veins by hydrothermal processes. In these rocks it often occurs as a gangue mineral associated with metallic ores. Fluorite is also found in the fractures and cavities of some limestones and dolomites. It is a very common rock-forming mineral found in many parts of the world. In the mining industry, fluorite is often called “fluorspar.”
Physical Properties of Fluorite
Chemical Classification Halide
Color Typically purple, green, and yellow. Also colorless, blue, red, and black.
Streak White
Luster Vitreous
Diaphaneity Transparent to translucent
Cleavage Four directions of perfect cleavage
Mohs Hardness 4
Specific Gravity 3.2
Diagnostic Cleavage, hardness, specific gravity, color
Properties
Chemical CaF2
Composition
Crystal System Isometric
Uses Numerous uses in the metallurgical, ceramics, and chemical industries. A
source of fluorine, hydrofluoric acid, metallurgical flux. High-clarity pieces are used to make lenses for microscopes, telescopes, and cameras.
Physical Properties of Fluorite
Fluorite is very easy to identify if you consider cleavage, hardness, and specific gravity. It is the only common mineral that has four directions of perfect cleavage, often breaking into pieces with the shape of an octahedron. It is also the mineral used for a hardness of four in the Mohs Hardness Scale. Finally, it has a specific gravity of 3.2, which is detectably higher than most other minerals.
Although color is not a reliable property for mineral identification, the characteristic purple, green, and yellow translucent-to-transparent appearance of fluorite is an immediate visual clue for the mineral.
Fluorite Occurrence
Most fluorite occurs as vein fillings in rocks that have been subjected to hydrothermal activity.
These veins often contain metallic ores which can include sulfides of tin, silver, lead, zinc, copper, and other metals.
Fluorite is also found in the fractures and vugs of some limestones and dolomites. Fluorite can be massive, granular, or euhedral as octahedral or cubic crystals. Fluorite is a common mineral in hydrothermal and carbonate rocks worldwide.
Fluoride products
Most people are familiar with fluoride products used in the prevention of tooth decay. Fluoride is added to drinking water as a systemic fluoride therapy and added to toothpastes, mouthwashes and dental rinse as a topical fluoride.
Uses of Fluorite
Fluorite has a wide variety of uses. The primary uses are in the metallurgical, ceramics, and chemical industries; however, optical, lapidary, and other uses are also important.
Fluorspar, the name used for fluorite when it is sold as a bulk material or in processed form, is sold in three different grades (acid, ceramic, and metallurgical).
Acid Grade Fluorspar
Acid grade fluorspar is a high-purity material used by the chemical industry. It contains over 97% CaF2. Most of the fluorspar consumed in the United States is acid grade even if it is used in lower grade applications. It is used mainly in the chemical industry to manufacture hydrofluoric acid (HF). The HF is then used to manufacture a variety of products which include: fluorocarbon chemicals, foam blowing agents, refrigerants, and a variety of fluoride chemicals.
Ceramic Grade Fluorspar
Ceramic grade fluorspar contains between 85% and 96% CaF2. Much of this material is used in the manufacture of specialty glass, ceramics, and enamelware. Fluorspar is used to make glazes and surface treatments that produce hard glossy surfaces, opalescent surfaces, and a number of other appearances that make consumer glass objects more attractive or more durable. The non-stick cooking surface known as Teflon is made using fluorine derived from fluorite.
Metallurgical Grade Fluorspar
Metallurgical grade fluorspar contains between 60 and 85% CaF2. Much of this material is used in the production of iron, steel, and other metals. Fluorspar can serve as a flux that removes impurities such assulfur and phosphorous from molten metal and improves the fluidity of slag. Between 20 and 60 pounds of fluorspar is used for every ton of metal produced. In the United States many metal producers use fluorspar that exceeds metallurgical grade.
Optical Grade Fluorite
Specimens of fluorite with exceptional optical clarity have been used as lenses. Fluorite has a very low refractive index and a very low dispersion. These two characteristics enable the lens to produce extremely sharp images. Today, instead of using natural fluorite crystals to manufacture these lenses, high-purity fluorite is melted and combined with other materials to produce synthetic “fluorite” lenses of even higher quality. These lenses are used in optical equipment such as microscopes, telescopes, and cameras.
Lapidary Grade Fluorite
Specimens of fluorite with exceptional color and clarity are often used by lapidaries to cut gemstones and make ornamental objects. High-quality specimens of fluorite make beautiful faceted stones; however, the mineral is so soft and cleaves so easily that these stones are either sold as collector’s specimens or used in jewelry that will not be subjected to impact or abrasion. Fluorite is also cut and carved into ornamental objects such as small figurines and vases. These are often treated with a.
Fluorspar
By Michele E. McRae
Domestic survey data and tables were prepared by Samir Hakim, statistical assistant, and the world production table was prepared by Lisa D. Miller, international data coordinator.
